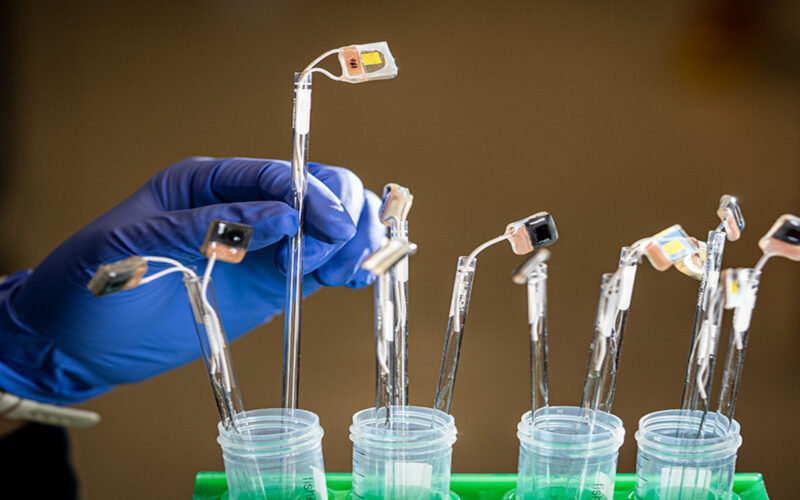
Researchers have made an outstanding step towards sustainable energy solutions after they developed an artificial leaf capable of converting carbon dioxide into liquid fuels and other valuable chemicals using sunlight alone.
The team of experts from the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and their international collaborators brought science one step closer to replicating the incredible productivity of natural photosynthesis, but with the added potential for industrial-scale application.
By harnessing copper’s catalytic power alongside perovskite – a calcium titanium oxide mineral widely used in solar panels – they developed a self-contained system that captures sunlight and converts carbon dioxide (CO₂) into carbon-carbon (C2) molecules.
These C2 molecules – a green, gaseous inorganic chemical also known as diatomic carbon – are essential building blocks for various industries, including fuel production and plastics.
Efficient carbon conversion
A part of the Liquid Sunlight Alliance (LiSA), a Department of Energy-funded initiative led by Caltech in close partnership with Berkeley Lab, the project draws on the expertise of over 100 scientists from institutions including SLAC, the National Renewable Energy Laboratory, UC Irvine, UC San Diego, and the University of Oregon. To further its mission, LiSA aims to advance technologies that turn sunlight, CO₂, and water into fuels.
“Nature was our inspiration,” said Peidong Yang, PhD, a senior faculty scientist in Berkeley Lab’s material sciences division and UC Berkeley professor of chemistry and materials science and engineering.
“We had to work on the individual components first, but when we brought everything together and realized that it was successful, it was a very exciting moment.”

Credit: Marilyn Sargent/Berkeley Lab
Yang and his team followed the natural processes in the leaf of a plant to build the postage stamp-sized device that mimics the way green plants harvest energy from the sun. However, instead of chlorophyll – a natural compound found in green plants that gives them their color and plays a key role in the process of photosynthesis – the scientists used lead halide perovskite photoabsorbers to capture sunlight.
These were paired with finely engineered copper electrocatalysts – designed to resemble tiny flowers – to drive chemical reactions. The electrocatalysts were reportedly inspired by enzymes that regulate photosynthesis in nature. Yang explained how each individual component of a leaf’s photosynthesizing elements had to be carefully replicated and refined.
C2 molecule production
Even though other experiments have achieved artificial photosynthesis using biological materials, Yang’s team chose copper, an inorganic material with lower selectivity for desired chemical reactions than biological catalysts. However, copper offers significant advantages such as greater durability, stability, and longer operational life, all critical for scaling up future applications.
While LiSA experts developed the device’s cathode and anode components, the integration with metal contacts was carried out using instruments at Berkeley Lab’s Molecular Foundry. During testing, a solar simulator replicating continuous sunlight demonstrated that the system could efficiently convert CO₂ into carbon-carbon (C2) molecules, essential building blocks for a wide range of industries.

Credit: Marilyn Sargent/Berkeley Lab
Building on earlier advances, the scientists successfully triggered an organic oxidation reaction in the photoanode chamber while producing C2 molecules in the photocathode chamber. This achievement resulted in a realistic artificial-leaf architecture, condensed into a device about the size of a postage stamp, that converts CO₂ into C2 molecules using only sunlight.
The team said that the artificial leaf’s ability to produce C2 chemicals may soon lead to precursory ingredients for industries that manufacture essential products, from plastic polymers to fuels for heavy transport like airplanes, which remain difficult to electrify. Yang and his team hope to improve the system’s efficiency and expand the device’s size, moving closer to scaling the technology for practical use.